Making Switzerland's road traffic fit for the future calls for research, first and foremost. In the large-scale research facilities of PSI, chemists and engineers are investigating how to improve the efficiency of motors and reduce their emissions.
"The overall transportation system of Switzerland in 2040 is efficient in all aspects." The primary strategic goal of the Federal Department of the Environment, Transport, Energy and Communications (DETEC) sounds good. The subordinate Swiss Federal Office of Energy (SFOE) specifies that vehicular traffic should pollute the environment less and become more energy-efficient and climate-friendly. Switzerland has set an ambitious goal for itself: to be climate-neutral by 2050.
This is a major challenge. According to the most recent "microcensus" on mobility from 2015, every person living in Switzerland travels around 24,850 kilometres per year. A high number, which also includes trips abroad. In everyday life and within Switzerland, the average per person is nearly 37 kilometres per day – and rising.
According to the Federal Office for the Environment (FOEN), cars, trucks, and buses produce three-fourths of the greenhouse gas emissions in the transportation sector. From this it follows: Whether or not the nation achieves its goal depends heavily on the motors used in these modes of transportation. Their CO2 emissions must be radically reduced. This is precisely the starting point for researchers at PSI and other institutions.
In one study, Christian Bauer and Brian Cox of the PSI Laboratory for Energy Systems Analysis determined how much exhaust gas – greenhouse gases in particular – the different types of automobile motors produce today and how much they will produce in 2040 according to current trends. They considered the entire life cycle of the vehicles, from production through disposal. Diesel vehicles in particular have been criticised in recent years because of their especially high emissions of the harmful nitrogen oxides. The PSI research groups led by Davide Ferri and Maarten Nachtegaal have found out how these emissions can be significantly reduced. Already today, many diesel vehicles run with an additive called AdBlue, which is injected into the exhaust gas and there breaks down into ammonia. With the help of a catalyst, this reacts with the nitrogen oxides to form harmless nitrogen and water. This only works effectively, however, if the exhaust gases are at a temperature above 200 degrees Celsius. As a consequence, a lot of nitrogen oxide is left over in the first minutes of a drive and on cold winter days.
With the concentrated X-rays of the Swiss Light Source SLS, the researchers have examined the catalyst material (a copper-zeolite compound) and observed exactly what's going wrong: At low temperatures, too much ammonia reduces the effect of the copper. The PSI researchers' study found conclusively that, to optimise the effect, different amounts of ammonia should be injected depending on temperature and operating conditions. In this way, nitrogen oxide emissions can be reduced by up to 90 percent.
Nevertheless the diesel engine remains a motor with a negative impact on the climate – even more so the petrol engine. To drastically reduce this impact, new power system technologies are required, if possible completely without harmful emissions.
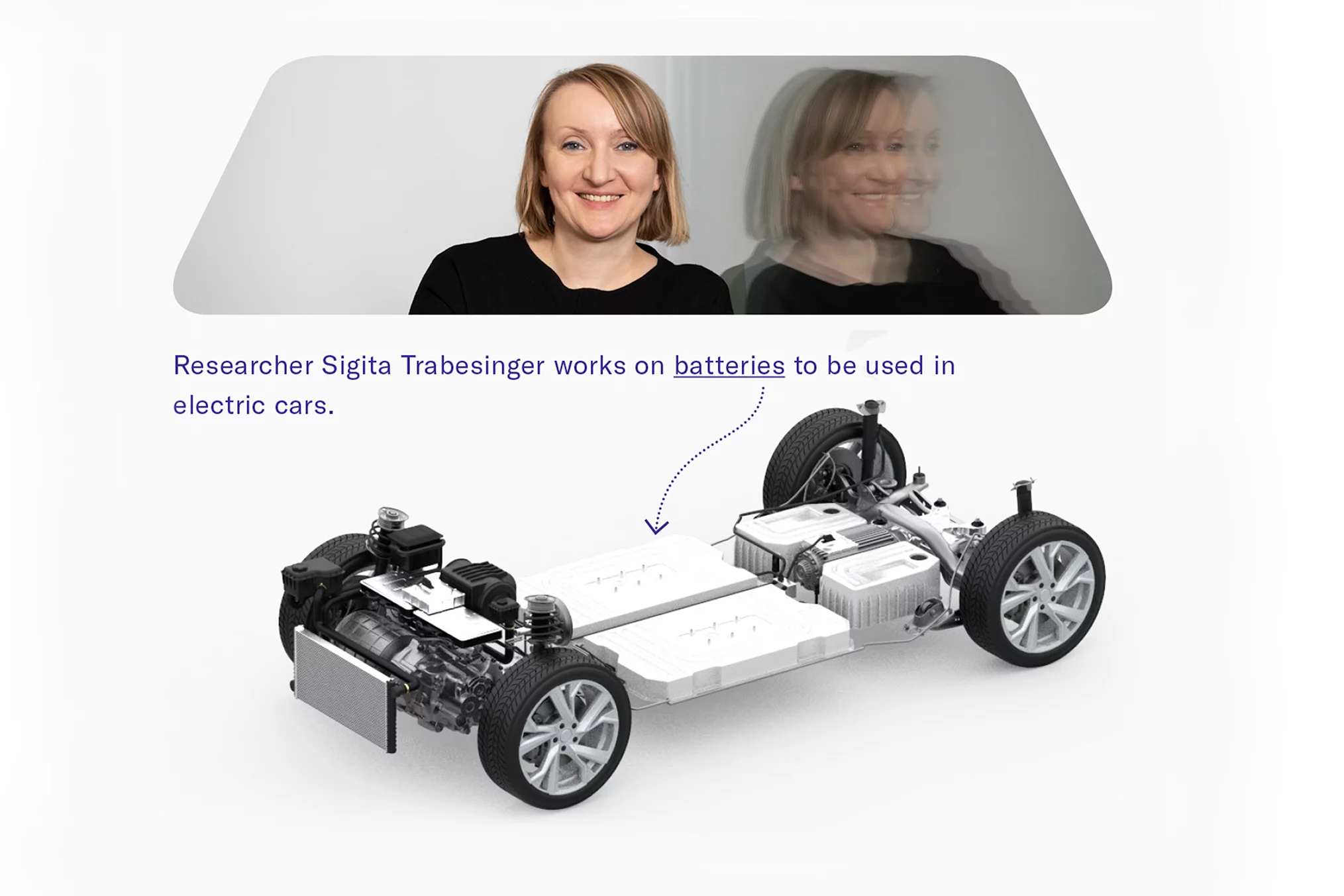
In this regard, electric motors are an obvious alternative. At PSI, intensive research is under way to improve their technology and particularly to increase their range, so that they soon could adequately replace diesel and petrol engines. Two questions PSI researchers seek to answer in this context concern energy storage for electric vehicles: Which electrolyte best transfers the charges in the battery? And how must the battery's electrodes be designed to achieve a maximum energy density –without increasing the risk of explosion? With these two challenges solved, conventional lithium-ion batteries would already be able to increase the range of electric vehicles.
A group led by PSI chemist Sigita Trabesinger, for example, is addressing the proportion of so-called transition metals in the positive electrode or cathode of the battery, together with the chemical company BASF. Trabesinger would like to know what effect changes in the composition would have on the stability and safety of the battery. The compound commonly used is called NCM, which consists of different proportions of nickel, cobalt, and manganese. "The goal is to increase the proportion of nickel and reduce the proportion of cobalt as far as possible", Trabesinger says.
That's because cobalt is not only toxic, but also rare and expensive, and it is mined mainly in the Congo under socially and ecologically questionable conditions. Besides that, a higher proportion of nickel offers a technical advantage – it increases the capacity of the cathode and thus the range of the electric vehicle. Ideally, the researchers would even like to completely eliminate the need for cobalt in the future.
However, NCM with more nickel and less cobalt tends to be unstable in air and to be more chemically reactive in the battery than desired.
Various tricks could still be used to stabilise the new materials: The addition of extremely small amounts of other elements is currently being tried, as is a targeted surface coating. With the ultra-precise light sources of PSI's large-scale research facilities, Trabesinger and her colleagues are investigating exactly why the electrodes become unstable without these tricks, and which of the changes are promising for success.
But maybe the future of the electric motor lies also in the so-called solid-state battery. Instead of the typical liquid electrolyte that transfers the charges between the electrodes, a solid-state battery contains a solid ceramic. Its advantage: It is less combustible than liquid electrolytes. This battery also can withstand high voltage and high temperatures without cooling – and thus can, theoretically, charge more rapidly and save the space normally required for the cooling device. And it also promises a higher energy density than present-day lithium-ion batteries. However, charging these batteries still takes a comparatively long time, because only a relatively low current can be put into them.
PSI researchers got to the bottom of this with so-called operando X-ray tomographic microscopy. "In principle, this works like computer tomography in a hospital," says beamline scientist Federica Marone. "However, the photon flux here at our large-scale research facility SLS is several orders of magnitude higher. This enables us to achieve the needed spatial and temporal resolution." Marone was able to observe with unprecedented precision how cracks form during charging in the ceramic, which in this case consisted of lithium sulfide and phosphorus sulfide: The lithium ions squeeze into the molecular lattice of tin pellets embedded in the ceramic, thereby increasing their volume by up to 300 percent. Marone was able to follow how the cracks propagated and disrupted the flow of ions between the electrodes. In fact, the cracks close again during discharge, since the solid electrolyte has a certain elasticity. But the limitation on charging remains. With this understanding, it is now easier to search for other electrolyte materials that react less strongly to the expansion of the tin pellets. Nevertheless, we're still a long way from fault-free mass production of solid-state batteries.
The situation looks different for fuel cells. In comparison to batteries, these power systems have a range advantage, although they are less energy-efficient. "The technology is mature, and there are no more major obstacles," says Thomas J. Schmidt, head of the Energy and Environment Research Division at PSI. "All that's lacking now is the political will."
Meanwhile, the fuel cell is being further optimised. Here too the researchers are using the unique imaging techniques that PSI offers with its large-scale research facilities. For a long time, the processes taking place inside the fuel cell were not understood in detail. Now, however, it is possible to look inside the cell while it's operating, with the help of neutron radiography and X-ray tomography using synchrotron radiation.
In a fuel cell, a reaction occurs that schoolchildren already learn about in chemistry lessons: The gases hydrogen and oxygen combine to form water and release energy in the process. This takes place on the two porous catalyst layers of the electrodes, which are separated by a membrane. The two gases must diffuse through these layers, andthe water formed must be removed through them as well. The latter is especially important for a fuel cell to work well. Otherwise, the water clogs the pores of the electrodes and thus blocks the gases. In winter, when water freezes and expands, there is even a risk of mechanical damage. Therefore, the PSI researchers are testing how the flows of water and gas could be optimised, for example, by changing the porous components in the cell.
Thanks to better water management and optimised catalysts, the next generation of fuel cells should not only be more durable, but also offer a higher current density. "The current density," according to Thomas J. Schmidt, "is particularly important for the flexibility of the power system. And this has a direct impact on the size of the fuel cell systems, and thus on their costs as well."
Advances such as these are finding applications, not least in a cooperation with the company Swiss Hydrogen (see 5232 issue 1/2018, page 18). It has upgraded conventional electric cars with fuel cells that charge the battery while driving and thus increase the range. Also, since 2017, a truck equipped with this kind of power system has been operating in the Coop transport fleet to test this application.
Natural gas is also a major topic when it comes to alternative ways to power our vehicles. Even fossil natural gas, which some cars already use today, is better than petrol or diesel fuel in terms of greenhouse gases. "Methane is the main component of natural gas and chemically has a more favourable ratio between hydrogen and carbon as well as higher knock-resistance than petrol or diesel," explains Oliver Kröcher, head of the Bioenergy and Catalysis Laboratory at PSI. This allows the gas in the motor to be compressed more and to burn more efficiently. Although the CO2 emissions are in fact lower, unburned methane must be removed from the exhaust. Catalysts required for this are being studied and developed in Kröcher's laboratory.
Gas engines that run on biomethane obtained from biomass – also known as "synthetic natural gas" (SNG) – could also become interesting. The carbon it contains was previously removed from the atmosphere, which means that at least the direct emissions of the vehicles are virtually carbon-neutral. Various possibilities for producing biomethane are being studied at PSI.
One possible approach is to ferment organic waste in biogas plants. In a long-term experiment, a relevant test system at PSI has already proven its suitability for everyday use. The process used is especially efficient because not only is methane obtained from the fermentation process, but carbon dioxide in the fermentation gases is also converted to methane with the help of added hydrogen (see 5232 issue 1/2018, page 16).
The overall process only makes ecological sense, though, if the added hydrogen is also produced with renewable energy – this applies here as well as in the case of hydrogen for fuel cells. By electrolysis, for example. PSI is also doing research on this: "We are developing suitable materials that ensure efficient electrolysis," says Felix Büchi, head of the Laboratory for Electrochemistry.
In some of these areas, PSI researchers share their expert knowledge with other Swiss institutes, including Empa, the Federal Laboratories for Materials Science and Technology. "Empa is a capable partner for us in many of our research projects," says PSI division head Schmidt. PSI and Empa are working together with other Swiss institutes and universities within the framework of competence centres on Heat and Electricity Storage as well as Mobility. As the name suggests, the latter focuses on the topic of efficient mobility.
Indeed, "efficiency" is the magic word in the further development of many alternative power systems for vehicles. The experts emphasise that you always have to keep an eye on the overall transportation system, and on cost-effectiveness: "After all, it is about getting from A to B in the most environmentally friendly and affordable way possible," says Serge Biollaz, head of the Thermochemical Processes Research Group at PSI. "To do this, we have to link many solutions together in a meaningful way. And we need not just one motor technology for the mobility of the future, but a wealth of options. Only then can we achieve the Swiss goals for an efficient and low-carbon transportation system."
Text: Jan Berndorff