Research in the radiation protection lab
Whether in muesli, lunch rolls or berry-based desserts, we take folic acid on board in our daily diet. This vitamin, also commonly known as the vitamin of life, is important for cell division and, therefore, growth. In future, however, the vitamin could take on another role, as cancer therapies that use folic acid are being developed at PSI. The principle is straightforward: tumour tissue grows rapidly and needs a lot of folic acid. Consequently, the vitamin, which is absorbed via folate receptors into the cells, can serve as a means of transport to carry pharmaceuticals into the tumour. Cristina Müller from the Center of Radiopharmaceutical Sciences is researching the treatment in question with radioactively marked folate compounds. “These enter the cell unimpeded like a Trojan horse then kill it with their radiation,” she explains.
Novel idea, new questions
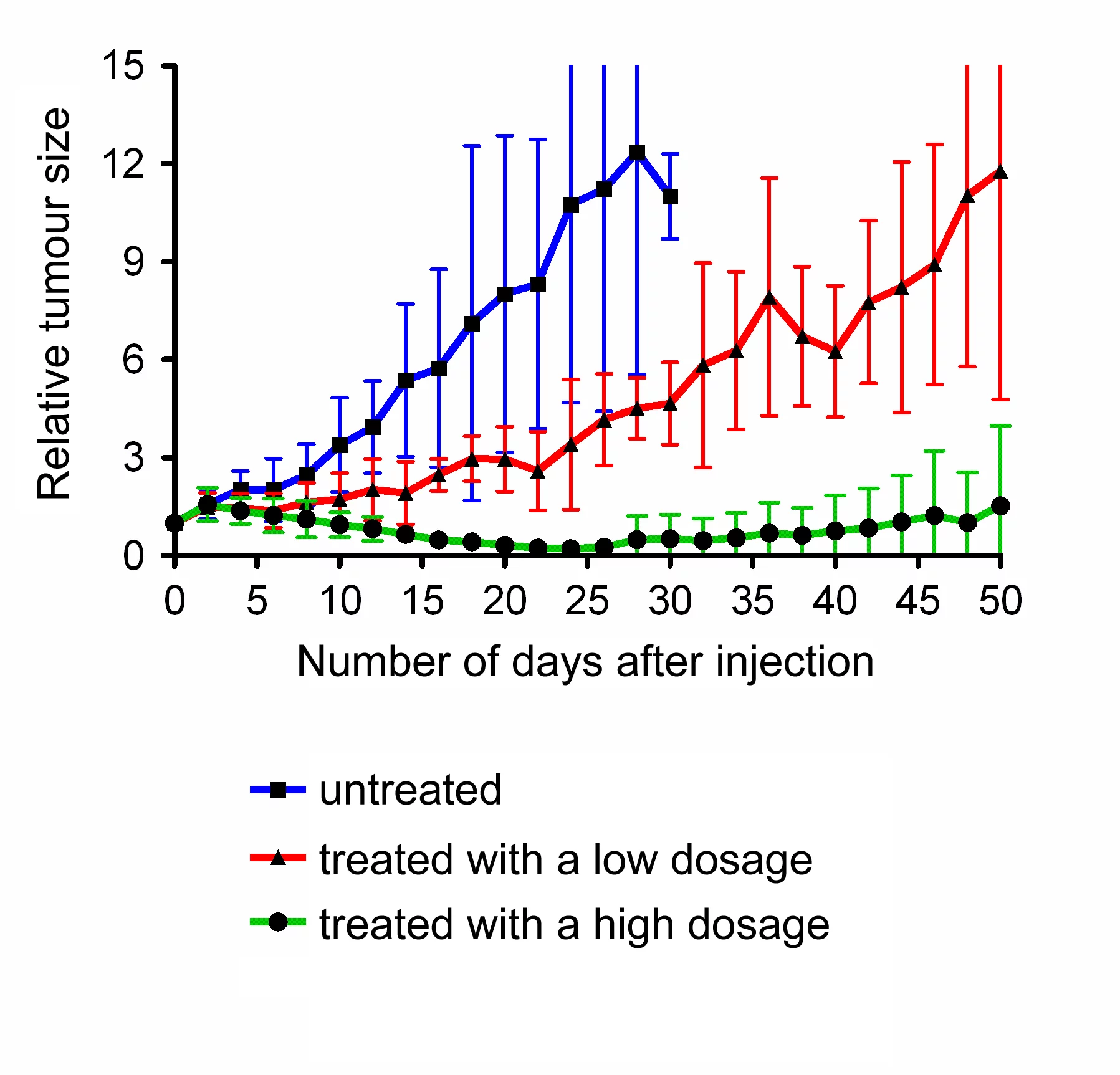
The pharmaceuticals researcher developed the method with the folic acid molecules, so-called folates, from scratch. The group recently became the first ever to test a new kind of radioactive folate in mice. Apart from folic acid that fits onto the receptor and the radioactive molecule – the actual medication – this variety also contains a docking site for a protein molecule in the bloodstream. As a result, the tri-construct binds to the protein in the blood and, thus, circulates longer in the body, which increases the absorption of the radiofolates in the tumour cells. “We were able to demonstrate a decrease in the tumours and even their disappearance in mice,” says Müller. Despite the initial successes, however, there are still a number of hurdles to overcome. “The problem is that renal tissue also contains many folate receptors and, thus, absorbs radiating radiofolates,” says Müller. Eventually, this leads to kidney damage. One possible solution that Müller is currently testing consists in saturating the kidneys with natural folates so that the radioactive form can no longer dock onto the receptors in the renal tissue.
Dosimeters a must
Müller’s research is conducted in a controlled zone at PSI with access only granted to people who are registered. They must wear a personal dosimeter, which measures the radiation level and triggers an alarm if the exposure exceeds the permissible level. For Müller, this is all in a day’s work. “Really, we’re a completely normal lab,” she remarks as she slips into her lab coat, slides the dosimeter into her breast pocket and adds: “Our work isn’t dangerous, just so long as everyone takes radiation seriously.” As you can’t see, hear or smell radioactivity, you have to be very careful indeed. “That’s why new staff members need intensive induction before handling radioactivity and the corresponding rules of conduct become second nature,” explains Müller, who is currently supervising three doctoral students, one masters student and one laboratory technician.
Decaying work material
Radioactivity is a perfectly natural process: during the decay of unstable atomic nuclei, energy is released in the form of radiation. As the material radiates its quantity diminishes (known as “decaying”). “The actual challenge in our research is planning the experiments.” The radioactive materials are either produced at PSI or ordered from abroad. Lutetium-177, for instance, is brought here from Germany. When the delivery arrives in the radiation-diminishing lead receptacle, the experiments have to be ready for labeling – before the delivery decays.
In order to attach the radioactive substances to the folates, the two components are mixed together. In vivo experiments only take place after extensive preliminary tests with cells. A mouse is injected with the radiofolate compound to be studied and the animal is scanned with a so-called small-animal SPECT camera, a device that detects the radiation given off by the lutetium-177. The resulting image reveals where the radiofolates have accumulated. In order to find out how the radiation damages the kidneys, they are subsequently removed and studied using thinly sliced tissue.
Research for practice
Cristina Müller removes her lab coat, steps on a device measuring radioactive contamination at the exit. The hand-foot monitor but displays “not contaminated” indicating that Müller can leave the lab unconcerned. “It’s bound to be years before we can offer radiotherapy for the clinic,” says Müller. Until then, Müller is looking for a suitable combination of an optimum absorption of folates in the tumour and the protection of the kidneys. It is important to Müller that her work is meaningful. “The prospect of being able to help cancer patients with my research somewhere down the line is a huge motivation.”
Text: Simone Nägeli
Original Publication
-
Müller C, Struthers H, Winiger C, Zhernosekov K, Schibli R
DOTA conjugate with an albumin-binding entity enables the first folic acid-targeted 177Lu-radionuclide tumor therapy in mice
Journal of Nuclear Medicine. 2013; 54(1): 124-131. https://doi.org/10.2967/jnumed.112.107235
DORA PSI