Diffusion and growth, on surface chemistry and supra-molecular chemistry are of profound importance for the functionalization of surfaces and for creating novel surface supported low dimensional materials and atomic scale switching devices. Fast, highly directive reactions with small functional units serving as reactants, so called “click” reactions, are well established in nature’s bio‐ chemistry and are particularly useful also to design specific ad‐surface molecular layers in subse‐quent reactions. Electron and spin-functional molecules have been synthesized with increasing complexity. The understanding of their aggregation and supramolecular assembly, however, is just at its start: The experience gathered and reaction mechanisms derived for “in‐solution” assemblies are not necessarily transferrable to solution‐free processes involving functional molecules at surfaces.
In order to control on‐surface assembly and reactions, it is possible to modify either the reactants or the substrate it‐self [1]. The former can be changed by, for example, replacing substituents [2], while the latter can be modified by several e.g. chemical methods as well as varying the number of active sites by changing roughness. The approach we chose in our study [3] involves modifying the reactive surface with an atomically thin layer of adsorbates and investigating its influence on the on‐surface metallation reaction of a 2HTPP porphyrin.
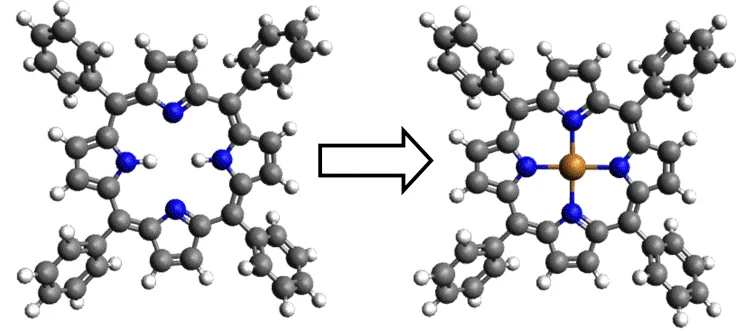
Currently we are investigating the influence of other adsorbate‐induced (e.g. N and Cl) surface super‐ structures on the availability of reactive metal atoms by studying the porphyrin metalation. We are able not only to promote a reaction, but also inhibit it completely. Also we have exploited the earlier demonstrated principles of programmed supramolecular and chemical assembly for the formation of 2D free standing layers from engineered building blocks [4].
One of the surface science’s quests is to create 2D nanomaterials with new and more advanced functionalities in a defect free way. Scanning‐probe repositioning techniques allow for creation of well‐ defined, functional structures. Such structures however, do not meet other requirement valuable for future devices – scalability, since creation of large (few hundred nanometers) structures would be very time‐consuming. Our supra-molecular approach, however, allows for fabrication of extended 2D layers with potentially interesting electronic properties by means of self‐assembly.
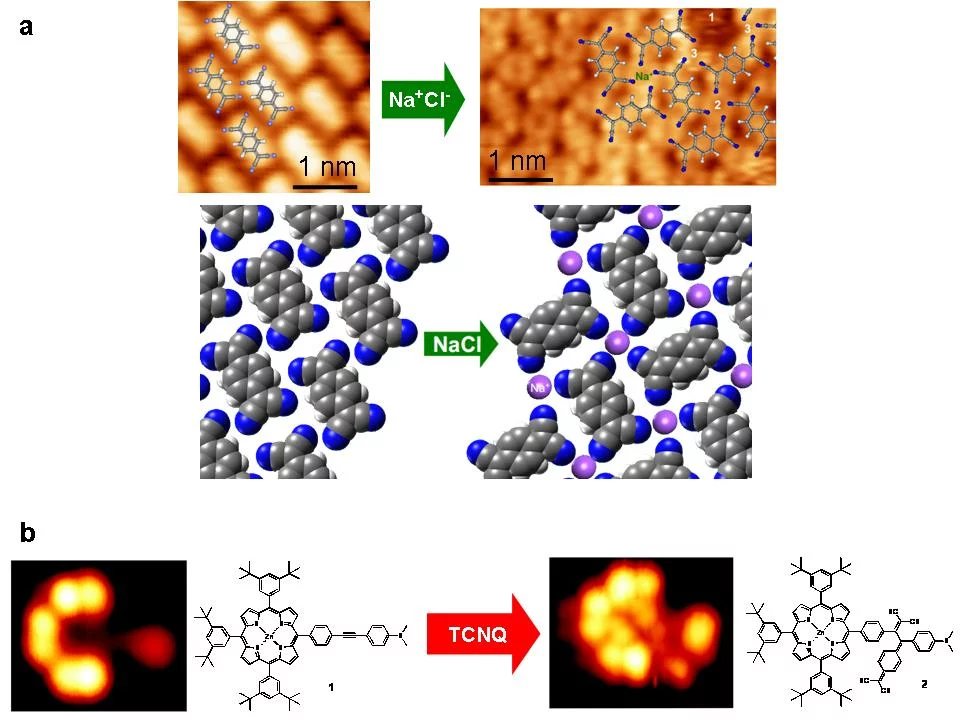
tetracyano‐p‐quinodimethane (TCNQ) and NaCl, both sublimed onto a Au(111) surface in a sub‐monolayer regime, has been studied. STM has been used to probe self‐assembly of TCNQ/Au(111) and NaCl+TCNQ/Au(111) (Fig. 1a). In the former case, self assembly is mainly ascribed to H‐bonding of the cyano groups [5]. In the latter, arrangement of TCNQ is significantly influenced by the addition of NaCl. [6].
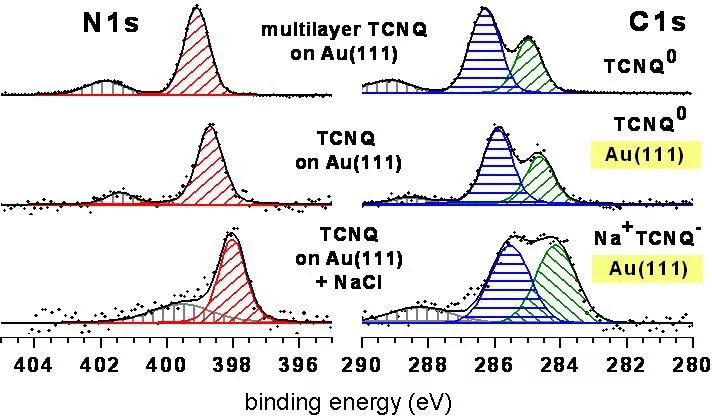
XPS data (Fig. 2) indicate the reduction of TCNQo to TCNQ‐ due to an on‐surface CT reaction with NaCl. Further photoelectron spec‐ troscopy studies prove that the CT occurs between TCNQ and NaCl without involving the Au(111) substrate. As a result, Cl2 gas is re‐ leased. Thanks to this reaction, extended 2D molecular structure could be obtained.
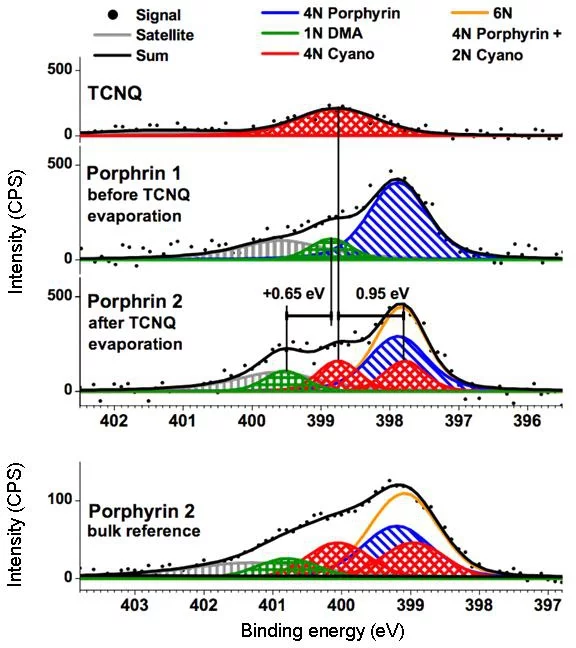
Another on‐surface chemical reaction [7] was observed for a zinc porphyrin (ZnTPP, see Fig. 1b) on Au(111) substrate. When exposed to TCNQ molecule at room temperature, a covalent bond between the two molecules is formed and a new molecule (Fig. 1b) arises. The XPS (Fig. 3) data unequivocally prove the covalent bond formation and creation of the ZnTPP‐TCNQ complex.
References
[1] Molecular Chessboard Assemblies Sorted by Site-Specific Interactions of Out-of-Plane d‑Orbitals with a Semimetal Template
A. Wäckerlin, S. Fatayer, T. Nijs, S. Nowakowska , F. Mousavi, O. Popova, A. Ahsan, T. A. Jung and C. Wäckerlin.
Nano Lett. 2017, 17, 1956−1962
[2] Two-Dimensional Supramolecular Electron Spin Arrays.
C. Waeckerlin et al.
Adv. Mater.2013, 25, 2404–2408
[3] Porphyrin metalation providing an example of a redox reaction facilitated by a surface reconstruction
J. Nowakowski, C. Wäckerlin, J. Girovsky, D. Siewert, T. A. Jung, N. Ballav
Chem. Commun. 49, 2347 (2013)
[4] Supramolecular architectures of molecularly thin yet robust free-standing layers.
M. Moradi et al.
Sci. Adv. 2019;5: eaav4489
[5] Assembly of 2D ionic layers by reaction of alkali halides with the organic electrophile 7,7,8,8-tetracyano-p-quinodimethane (TCNQ)
C. Wäckerlin, C. Iacovita, D. Chylarecka, P. Fesser, T. A. Jung, N. Ballav
Chem. Commun. 47, 9146 (2011)
[6] Structure and electronic configuration of tetracyanoquinodimethane layers on a Au(111) surface
I. Fernandez-Torrente, K. J. Franke, J. I. Pascual
Int. J. Mass Spectrom. 277, 269 (2008)
[7] Visualizing the Product of a Formal Cycloaddition of 7,7,8,8-Tetracyano-p-quinodimethane (TCNQ) to an Acetylene-Appended Porphyrin by Scanning Tunneling Microscopy on Au(111)
P. Fesser, C. Iacovita, C. Wäckerlin, S. Vijayaraghavan, N. Ballav, et al.
Chem. Eur. J. 17 : 19, 5246 (2011)