Most cancer cells carry unique receptors on their surface. Because the receptors extend into the cell's interior, they act as intermediaries between the outside and the inside. Chemotherapeutic drugs that dock on the exterior trigger a cascade of biochemical reactions inside the cell. At the end of this process, the cancer cells should die off. Researchers at the Paul Scherrer Institute PSI are now investigating a new method that would not only attach radioactive substances to the outside of a cancer cell, but also would channel them right into the cell's nucleus. Thus the radiation source would remain inside the cell and work in a more targeted way, by getting closer to the genetic information. If the suitable radioactive compounds can be found, this method has the potential to help with several kinds of cancer in the future.
One of the most important goals in cancer therapy is to strike at the heart
of the camouflaged cancer cells – that is, in the nucleus. In cancer cells, pathologically mutated DNA ensures that cell division occurs more rapidly and more frequently than in normal cells. Many cytotoxins used in chemotherapy against cancer manage to penetrate to the cell nucleus and attack precisely those processes that are important for cell division. Others interfere with the metabolism of tumour cells, thereby impeding their growth. Thus they all operate within the cell, and particularly at the time when it is dividing. Many cytotoxins, however, are non-specific and also attack other tissues of the body that renew themselves frequently, such as hair or mucous membranes. Healthy cells can also be damaged by conventional, externally applied radiation treatments, because the ionising radiation hits all cells within the treated area.
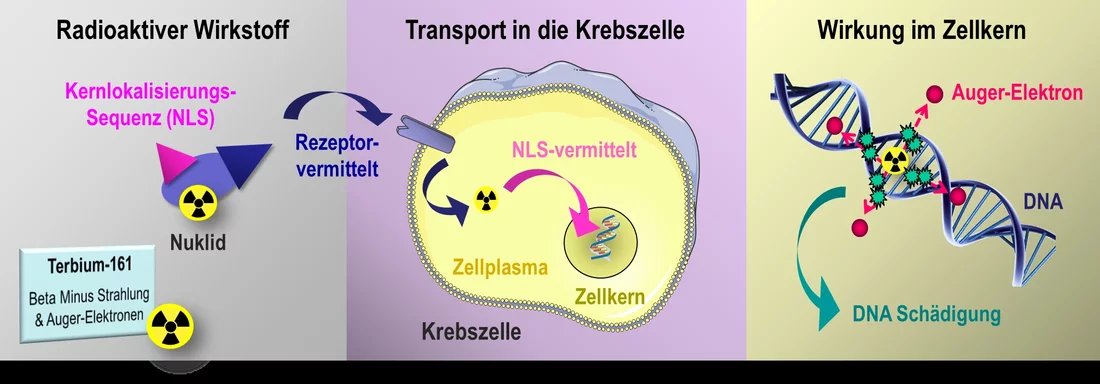
In contrast, the action of radioactive pharmaceuticals injected into the patient's bloodstream is very specific. They consist of a complex of several molecules, most often including a peptide and a radioactive metal atom. With the peptide, they latch onto the corresponding receptors on the outer surface of the tumour cell. The radionuclide coupled to it – the radioactive part of the drug – either remains lodged outside on the receptor or is transported into the cytoplasm. While the radionuclide decays, it sends out radiation that has a range of a few millimeters. This radiation penetrates the interior of the cell, including the nucleus, and also reaches neighbouring cells. The nuclide itself, however, never gets as far as the nucleus. That is prevented by a protective membrane, the nuclear envelope, which surrounds it.
Two ways to hit the target
Together with her team, pharmaceutical scientist Cristina Müller of the Center for Radiopharmaceutical Sciences (CRS) at the PSI is now investigating a possible avenue to further improve the effectiveness of radioactive nuclides in the cell nucleus. She does this in two ways: On the one hand, she is using a new radionuclide that has not previously been applied in the treatment of patients; on the other hand, she is coupling the radioactive substance with an additional peptide molecule known as an NLS, a nuclear localisation signal. This NLS can pass through the nuclear envelope by binding to transport molecules and thus can channel any substances desired into the cell nucleus.
For her studies, Müller uses a radionuclide of the element terbium, one of the rare-earth metals. Terbium is particularly interesting to her because it has four different radioactive nuclides, which emit different types of radiation. They can cover the whole spectrum of diagnostics and treatment in nuclear medicine. Despite that, the development of clinical applications is still at an early stage, because a few of these nuclides are very difficult to produce. The isotope terbium-161, with which Müller is currently working, can be produced at the PSI. It is made there, through a collaboration between the CRS and the Laboratory for Radiochemistry at the neutron source SINQ, from the element gadolinium.
Direct attack inside the cell nucleus
Terbium-161 is a unique nuclide,
Müller enthusiastically explains. It sends out two kinds of radiation, each of which has a different effective range. The two radiation types are complementary and thus can be used together in cancer therapy.
One of these radiation types, beta-minus decay, has a range of a few millimeters. This penetrates the entire cell, and hundreds of neighbouring cells as well.
At the same time, terbium-161 emits so-called Auger electrons. Their range, less than one micrometer, is much smaller than the diameter of a cell. They would be of little use on the outside of a tumour cell. Inside the cell nucleus, though, they can damage the DNA strands so that the cell will not be capable of dividing. In our experiments with cell cultures, we have now shown that this principle really works, and the cells exhibit more double-strand breaks,
Müller adds, but only if we have coupled the radioactive substance with an NLS.
For comparison, Müller and her group have investigated radioactive molecular complexes that also contain terbium but no NLS. These have in fact killed off tumour cells, but the effect was many times stronger with an NLS. This will be important later on, for the treatment of patients,
Müller says. If you don't need to administer so much radioactivity, the radiation exposure can be reduced. However, we still need to carefully study other possible side-effects.
Universal principle promises versatile applications in cancer therapy
Müller is convinced that it will be possible in the future to treat certain types of cancer more effectively and with fewer side-effects through a suitable radionuclide therapy than with chemotherapy. Thus terbium-161, given the very short range of its Auger electrons, would be especially well suited to killing individual cancer cells scattered throughout the body, or to killing clusters of cells. Add to this that the beta-minus radiation of this nuclide and its half-life are comparable to those of the radionuclide lutetium-177. Lutetium-177 is already in use today for treatment of cancer patients. In contrast to lutetium-177, though, terbium-161 has the advantage of a double action with its two types of radiation – and that without causing additional side-effects in healthy organs. This Müller has shown in a recently published study using the kidney tissues of mice.
Despite the great potential of the radioactive pharmaceuticals, many years of development work lie ahead. The researchers must find out which receptors on which cancer cells are best suited for occupation by a radioactively tagged molecule. This should be taken up in such a precisely targeted way that no other cells will be damaged. Also, the link between the radioactive nuclide and the biomolecule must fit in such a way that the NLS can still be attached. Therefore Müller is meticulously investigating all of these individual parameters. With our research, we want to achieve an optimisation of the radionuclide therapy on two levels, in that we on the one hand use a more effective nuclide and on the other hand deliver this nuclide to the site where it works best,
Müller emphasizes. We want to exploit all of the potential that a nuclide such as terbium-161 offers.
Text: Sabine Goldhahn
Information box: Beta-minus decay vs. Auger electrons
The radioactive nuclide terbium-161, which researchers at the PSI want to investigate for use in cancer treatments, produces two kinds of radiation: beta-minus decay and Auger electrons. Physically, both types of radiation consist of electrons, but each has a different origin in the atom and a different kinetic energy. The electrons of beta-minus radiation are generated when a neutron collides with the atomic nucleus and is transformed into a proton and an electron. These electrons have a high kinetic energy and for that reason a relatively large range of action in the tissue.
Auger electrons on the other hand have their origin in the electron shell that surrounds the atomic nucleus, from which they are ejected through a complex process. Their energy and thus their range in the tissue are significantly smaller than those of electrons generated through beta-minus decay. While the beta-minus radiation is already used for medical purposes with patients, up to now Auger electrons are only in use under laboratory conditions. For more on the Auger process, see Wikipedia.
Additional information
- The article
Targeting cancer
provides an overview of the work of the Center for Radiopharmaceutical Sciences. - The articles
Developing a new drug against thyroid cancer
andMedicines made to order with pinpoint precision
describe the development and production of a drug at the Center for Radiopharmaceutical Sciences. - The article on how PSI researchers are developing efficient procedures for producing radionuclides used in medical diagnostics:
Designer nuclide for medical applications
. - How researchers at PSI produce radionuclides:
In the focus of the protons
.
Contact
PD Dr. Cristina Müller, leader of the Nuclide Chemistry Group in the Center for Radiopharmaceutical Sciences at the Paul Scherrer Institute PSI, ETH Zurich, and the Zurich University HospitalPaul Scherrer Institute, 5232 Villigen PSI, Switzerland
Telephone: +41 56 310 44 54, e-mail: cristina.mueller@psi.ch