Researchers at the Paul Scherrer Institute PSI have developed a drug to trace and treat a particularly malignant strain of thyroid cancer more effectively. They use a protein, which resembles the body’s own hormone (gastrin), and add a radioactive substance. Due to its shape, the protein molecule that is radioactively marked as a result can dock onto suitable receptors on the surface of tumour cells based on the lock-and-key principle. The radiation from the radioactive substance can then destroy the tumour, while largely sparing the surrounding tissue on account of its short range. One advantage of the new drug is that it can be used to treat a strain of thyroid cancer where the established treatment with radio-iodine is ineffective. The researchers at PSI have developed the new drug to such an extent that an initial study conducted on cancer patients at the University Hospital Basel can now get underway.
Medullary thyroid carcinoma is the third most common strain of thyroid cancer. Although, at less than ten per cent of all thyroid carcinomas, it is fairly rare, it ranks among the especially aggressive strains as it readily metastasises. Around a quarter of these tumours are hereditary, which means that sometimes children or young adults are even affected. The first manifestations of the disease are a lump in the throat and difficulty swallowing, eventually followed by hoarseness and respiratory problems. In the early stages, the patient can be cured by a complete surgical removal of the thyroid and subsequent chemotherapy. Once the tumour has metastasised, however, it is virtually incurable,
explains Martin Béhé, a chemist from the Center for Radiopharmaceutical Sciences at the Paul Scherrer Institute PSI. Although a patient can survive for eight to fifteen years, the tumour or its metastases produce hormones that cause side effects, such as high blood pressure and severe diarrhoea, which can seriously affect the patient’s quality of life.
The reason for the hormone production lies in the type of tumour cells. Unlike with the more common strains of thyroid cancer, medullary thyroid carcinoma does not originate from the typical iodine-storing tissue in the thyroid, which is why it cannot be treated with the otherwise efficacious radio-iodine therapy. Instead, it stems from the so-called c-cells. These are found in the connective tissue of the thyroid and produce the hormone calcitonin, which is involved in calcium phosphate metabolism. The c-cells carry a molecule on their surface, which normal thyroid cells do not have: the cholecystokinin-2 (CCK2) receptor where, among other things, the body’s own hormones cholecystokinin and gastrin bind. As an especially large number of such CCK2 receptors are found in around 92 per cent of all medullary thyroid carcinomas, this molecule is the ideal application point for targeted therapy – provided a substance can be found that has a similar chemical structure to gastrin and, thus, also binds to the CCK2 receptor. Such a specific molecule can be coupled with a suitable radioactive substance and the radioactivity, thus, transported directly to individual cancer cells,
says Béhé. The drug can then dock there and destroy the cancer with its radiation,
he explains.
EU-funded project
In order to find a suitable substance, Béhé and fellow European scientists teamed up to test twelve possible radioactively-marked drugs under the EU-funded initiative COST (European Cooperation in Science and Technology). The aim was to discover any substance that binds specifically to the CCK2 receptor, destroys as many tumour cells as possible and causes the least side effects. After several years of searching, Béhé and his team at PSI eventually struck gold: they combined a mini-gastrin (PSIG-2), which is smaller than a normal gastrin molecule, with the radionuclide lutetium-177 and dubbed the new substance 177LU-PSIG-2.
Minor alteration with major impact
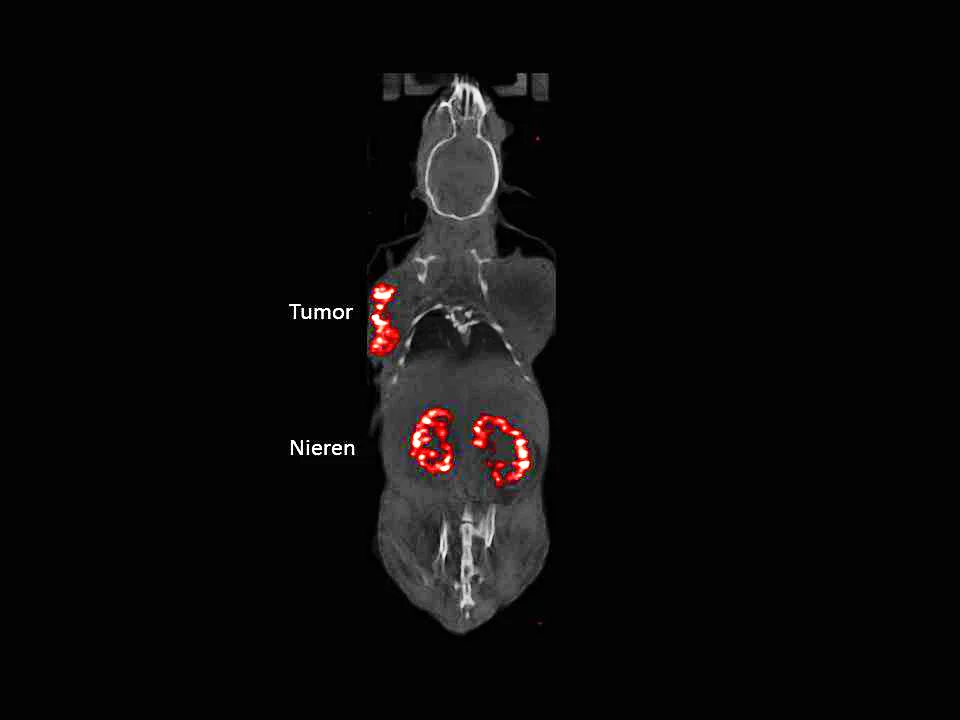
The path towards this radiopharmaceutical 177LU-PSIG-2 has been anything but straightforward, however. Initially, the researchers conducted experiments with a derivative of the hormone gastrin, which docked well to the CCK2 receptor. The initial results were promising: the medullary thyroid carcinoma regressed heavily in both animal tests and its initial use on humans. However, the new preparation did not just accumulate on the cancer cells, but also in the kidneys, where the substance remained for longer than desired and could, thus, have damaged the tissue. Consequently, the researchers went back to the drawing board. First of all, they had to get to the bottom of the heavy accumulation in the kidneys.
In their search, they discovered that certain amino acids – the glutamic acids, which make up many hormones and other protein molecules – were responsible. Six glutamic acids in the chemical structure of the preparation caused the kidneys to absorb the substance readily, therefore, the number of these components needed to be reduced in order to block this absorption. The result, however, did not go as hoped. The fewer glutamic acids the molecule had, the more susceptible it became to attacks from the body’s own enzymes, which rendered it inoperative. The six glutamic acids, thus, had to remain in the molecule. In order to prevent the heavy accumulation of the radioactive drug in the kidneys, the researchers had another trick up their sleeves: they replaced the natural glutamic acids with their synthetic counterparts, which go unrecognised by the body’s own enzymes and, therefore, cannot be destroyed, while the amount absorbed by the kidneys remains low.
Scientists use beta and gamma radiation
Finally, Béhé and his team had to solve another problem: the mini-gastrin PSIG-2 still contained the amino acid methionine, which can change its chemical make-up to such an extent that the gastrin derivative no longer binds well to the CCK2 receptor. The researchers substituted the methionine for the stable amino acid norleucine and, after more than a decade in the making, PSIG-2 was finally ready. What followed next was routine for the experts at PSI and only took another three years: they had to attach a suitable radionuclide to the mini-gastrin and opted for lutetium-177, as it emits both beta and gamma radiation. The beta radiation only travels a few millimetres in the body and, as soon as the radiopharmaceutical has docked, can destroy the tumour directly without damaging the surrounding tissue. The gamma radiation, on the other hand, leaves the body again and can be detected and measured with a gamma camera. Based on these readings, the camera produces an image that displays the accumulation of the radioactive substance in the body and is able to recognise the spread of the medullary thyroid carcinoma. We have developed the new drug to such an extent here at PSI that we can produce it in a standardised way, according to the pharmaceutical regulations,
beams Béhé. Now we have applied for approval from Swissmedic, Switzerland’s authority for therapeutic products, to use the drug on humans in a clinical trial by the end of the year.
As soon as the clinical trial with 177LU-PSIG-2 gets the green light, doctors from the Nuclear Medicine Clinic at the University of Basel will use it on test patients. This study on advanced-stage medullary thyroid cancer has already been authorised by the Swiss Ethics Committees and will be funded by Swiss Cancer League.
Text: Sabine Goldhahn
Additional information
- The article
Targeting cancer
provides an overview of the work of the Center for Radiopharmaceutical Sciences. - The article
Medicines made to order with pinpoint precision
describe the production of the drug mentioned above at the Center for Radiopharmaceutical Sciences. - A new method that would channel radioactive substances right into the cell's nucleus is described in the article:
Hitting cancer from the inside
. - The article on how PSI researchers are developing efficient procedures for producing radionuclides used in medical diagnostics:
Designer nuclide for medical applications
. - How researchers at PSI produce radionuclides:
In the focus of the protons
.
Contact
Dr Martin Béhé, Head of the Pharmacology Group at the Center forRadiopharmaceutical Sciences of the Paul Scherrer Institute PSI,
ETH Zurich and the University Hospital Zurich
Telephone: +41 56 310 28 17, e-mail: martin.behe@psi.ch