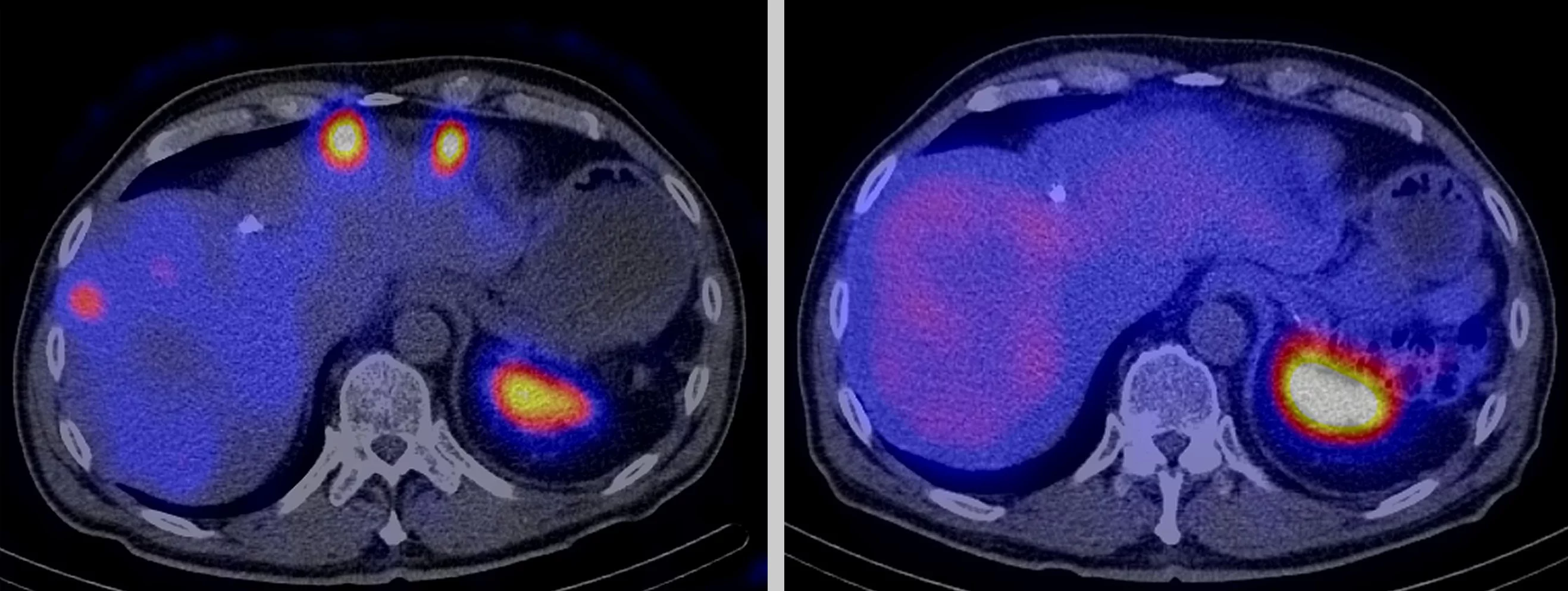
There are tumours where nothing seems to help: not chemotherapy, not external radiation therapy, not an operation. Often, they have already metastasised and can no longer be destroyed using conventional methods. The only option left here is internal radiotherapy with targeted radioactive drugs that strike directly at the heart of the disease. In order to make this possible, twenty specialists have been conducting research at the Centre for Radiopharmaceutical Sciences at the Paul Scherrer Institute PSI, a joint facility of PSI, ETH Zurich and the University Hospital Zurich.
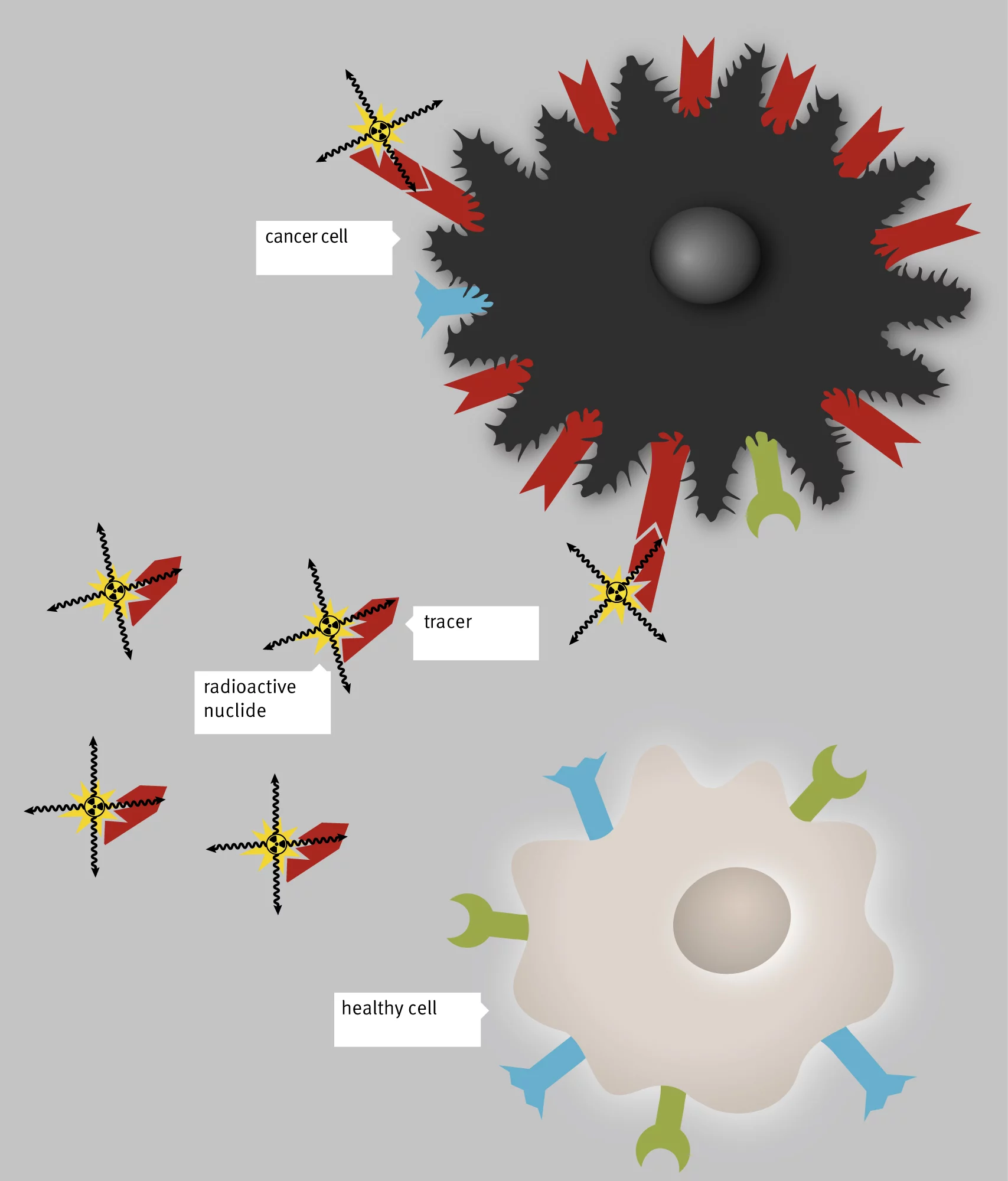
We can’t see, smell or feel radioactivity. Which is why many of us are afraid of this invisible energy and nuclear power stations and Fukushima immediately spring to mind. For cancer patients, however, radioactivity can save their lives. It helps doctors to trace and destroy a tumour and scattered cancer cells. They even use so-called radiopharmaceuticals, radioactive drugs with certain physical and chemical properties depending on the type of tumour cells and the application. Scientists also exploit the fact that tumour cells have special structures on their surface, where radiopharmaceuticals can dock if they contain the right molecule.
Every radioactive drug consists of one of these matching molecules, the so-called tracer
, and a radioactive isotope, which emits radiation. The substances simply seek out their target in the body themselves,
explains Christof Rottenburger, a nuclear physician at the University of Basel and a PSI project partner. They deposit the radiation locally and thus only cause minor side effects in general.
This is a major advantage for patients over chemotherapy and it also enables tumours that have formed many small metastases in the body and therefore cannot be operated on or irradiated from outside to be treated effectively and in a targeted manner.
Close collaboration with hospitals
Wherever doctors have run out of treatment options, that’s where our research comes in,
stresses Roger Schibli, Head of the Centre for Radiopharmaceutical Sciences (ZRW), a joint facility of PSI, ETH Zurich and the University Hospital Zurich. The collaboration with physicians is extremely important to him as they know best the diseases where nuclear medical procedures make sense. The researchers from ZRW then investigate the how and the what with. Five workgroups at PSI alone are attempting to develop the best radiopharmaceuticals for different cancer strains and other diseases. These drugs should be absorbed by the body quickly, bind specifically to the cells targeted and rapidly leave other tissue to avoid damaging it. They should also have a half-life that is long enough to guarantee a sufficient examination or treatment and give off radiation that can be recorded well by the cameras in the examination equipment. There is no such thing as the perfect radiopharmaceutical drug that works the same for all applications,
says Schibli. After all, there is a difference between radioactive drugs for diagnostic purposes and those to be used for treatment.
The differences here are greater that the similarities. For diagnostic questions, the researchers are on the lookout for radiopharmaceuticals where the radiation has such a large range that the distribution of the substance in the body can be measured externally and depicted using highly sensitive devices. Isotopes, which emit positron or gamma radiation, are just the ticket here. If a radioactive drug is to be used for therapeutic purposes, however, the main thing is that the radiation remains in the target area in the body and gives off a large amount of energy over a distance in the millimetre range, which enables a tumour to be destroyed. Radiopharmaceuticals that emit particle radiation, for example predominantly beta-minus or alpha radiation, are best suited for this purpose.
More than just physics
The road from the physics-based origin of a radiopharmaceutical drug to its optimum medical impact is long, takes years and often involves the work of all five research groups at PSI. The main challenge in our work is its interdisciplinary nature,
says Martin Béhé, Head of the Pharmacology research group at ZRW. If we want to achieve our goals, we have to consider biological, chemical, medical, pharmaceutical and physical aspects. Not only does everyone need a grasp of their specialist area, but also other fields. Sometimes it’s hard – but equally fascinating.
His work group primarily studies peptides, smaller proteins that are also found in healthy bodies. The researchers alter the components of the molecules or their structure so that they can attach a radioactive isotope, which transports its radiation directly to the cancer cells selected. This requires specialist expertise. On the one hand, we have to know what cells a tumour is composed of, how it developed and which symptoms it triggers,
explains Béhé. On the other hand, however, we also have to know how a substance we use to diagnose or combat a tumour behaves in the body, which metabolic pathways it passes through or how strongly and quickly it is absorbed by the kidneys and excreted again.
Besides his group, there is a second team of specialists: the group Nuclide Chemistry, which is specifically searching for new agents with a view to coupling them with radioactive molecules. The group primarily uses the vitamin folic acid, which cancer cells need to boost their growth. Many types of tumour carry folate receptors on their surfaces. As a matching chemical key is able to dock onto these receptors, this makes them the perfect target for radio-labelled folic acid molecules.
Production under scrutiny
Even if one of the two groups develops a radiopharmaceutical agent to such an extent that it can be used in humans for the first time, it will still take months or even years for it to reach the patient. First, the researchers have to demonstrate that the new substance is also a safe drug. This is where the workgroup Clinical Care at ZRW comes in, specialising in the highly sterile, standardised and therefore replicable production of radiopharmaceuticals in accordance with good manufacturing practice. However, the team also includes people with expertise in compiling all the extensive documentation that Swissmedic, the Swiss authority responsible for the authorisation and supervision of therapeutic products, requires for studies on patients and before a new radiopharmaceutical drug can be registered.
This does not just include the exact description of the tracer molecule, but also that of the radioactive isotope attached. Although there are hundreds of isotopes that come into question for use in humans, only a handful of them can be produced in a sufficient quantity and purity. First, we need to find a base material that can provide the desired radioactivity,
explains Schibli. Then,
he adds, we have to work out how we can separate the effective isotope from the base material and what happens to the by-products.
Two workgroups are responsible for these steps: Radionuclide Development, which is also part of PSI’s Laboratory for Radiochemistry, and Radionuclide-Production and Maintenance.
The researchers in both these groups have the biggest influence on the radioactive part of a substance, improve its production process and search for new isotopes. Thanks to the unique infrastructure at PSI, our accelerator facilities and neutron sources, we are also able to conduct research with isotopes that can only be produced here,
says Schibli. This opens up the possibility for us to keep developing better drugs that will one day help patients.
Although the radioactive drugs developed at ZRW have not been approved for use in everyday hospital life yet, the Centre assumes a pioneering role in their development. Its radiopharmaceutical drugs are prototypes, where all the important properties up to standardised production have been tested. Companies and authorities can use the results of this project as a benchmark when new radiopharmaceuticals are approved or new technologies are to be used.
Text: Sabine Goldhahn
Additional information
- The articles
Developing a new drug against thyroid cancer
andMedicines made to order with pinpoint precision
describe the development and production of a drug at the Center for Radiopharmaceutical Sciences. - A new method that would channel radioactive substances right into the cell's nucleus is described in the article:
Hitting cancer from the inside
. - The article on how PSI researchers are developing efficient procedures for producing radionuclides used in medical diagnostics:
Designer nuclide for medical applications
. - How researchers at PSI produce radionuclides:
In the focus of the protons
.
Contact
Prof. Dr Roger Schibli, Head of the Centre for Radiopharmaceutical Sciencesof the Paul Scherrer Institute (PSI), ETH Zurich and the University Hospital Zurich
Telephone: +41 56 310 28 37, e-mail: roger.schibli@psi.ch