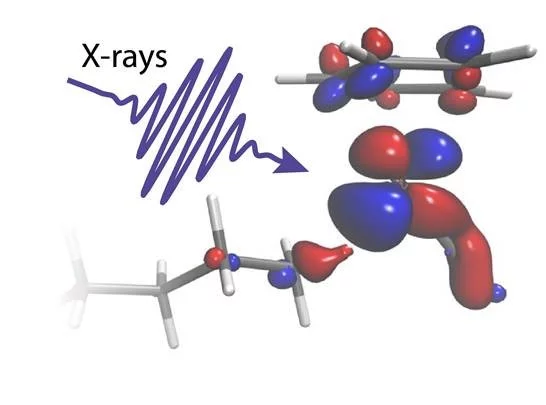
Short flashes of an unusual kind of X-ray light at SwissFEL and SLS bring scientists closer to developing better catalysts to transform the greenhouse gas methane into a less harmful chemical. The result, published in the journal Science, reveals for the first time how carbon-hydrogen bonds of alkanes break and how the catalyst works in this reaction.
Methane, one of the most potent greenhouse gases, is being released into the atmosphere at an increasing rate by livestock farming as well as the continuing unfreezing of permafrost. Transforming methane and longer-chain alkanes into less harmful and in fact useful chemicals would remove the associated threats, and in turn make available a huge feedstock for the chemical industry. However, transforming methane necessitates as a first step the breaking of a C-H bond, one of the strongest chemical linkages in nature.
Forty years ago, molecular metal catalysts were discovered that can easily split C-H bonds. The only thing found to be necessary was a short flash of visible light to “switch on” the catalyst and – bafflingly - the strong C-H bonds of alkanes passing nearby were easily broken almost without using any energy. Despite the importance of this so-called C-H activation reaction, it has remained unknown how that catalyst performs this function. Now, experiments at Swiss FEL and SLS have enabled a research team led by scientists at Uppsala University to directly watch the catalyst at work and reveal how it breaks the C-H bonds.
X-ray flashes at two complementary timescales
To study this process in action, the researchers used a technique known as time-resolved X-ray absorption spectroscopy. In their experiments, they triggered the reaction between a rhodium catalyst and an alkane, octane, using an optical laser. Like a high-speed camera, they then used extremely bright and short flashes of X-ray light to take snapshots of the bond breaking process. With this, they could follow the delicate exchange of electrons between the catalyst and a C-H group in octane as it breaks.
In the same way that an artist may first sketch an outline with pencil and then fill in the details with fine brush strokes, discovering the details of this bond breaking process needed two different speeds of flashes. The scientists first used the slower pulses of the SLS to map out the reaction from the beginning to the final C-H bond breaking after 14 nanoseconds (0.000000014 seconds). They then used the ultrafast pulses of the SwissFEL to study the initial light-induced activation of the rhodium catalyst that occurs within 400 femtoseconds (0.0000000000004 seconds).
“With this combination, we have a unique and efficient way to study how processes such as this happen from the excited state dynamics that happen on an ultrafast timescale to real catalyst, bond breaking chemistry that happens on slower timescales,” explains Camila Bacellar who is in charge of the Alvra experimental station at SwissFEL, where the experiments were carried out.
Tender X-rays: not too soft, not too hard but just right
The uniqueness that Bacellar refers to is not only the combination of different timescales, but the ability - at both SLS and SwissFEL - to access an unusual energy range: the tender X-ray regime. Tender X-rays lie at a sweet spot between the low energy (‘soft’) and high energy (‘hard’) X-rays typically used for research. This energy is perfectly suited to studying a range of elements – not in the least, rhodium - that play important roles in catalysis, battery research, photovoltaics, biology and environmental science.
“There are not many facilities that provide this range because it’s difficult to implement and requires extensive modifications of the beamline. But these energies are pretty important. They give us access to some elements that are widely available in nature,” says Thomas Huthwelker, who is responsible for the Phoenix beamline at the SLS. “These include sulfur, phosphorous, calcium and also many transition metals.”
The Phoenix beamline - designed for environmental and chemical science - is tailored to this regime. Recently, thanks to a collaboration with scientist Grigory Smolentsev, a mobile pump-probe setup, enables time-resolved measurements. This makes Phoenix the only synchrotron beamline in the world where time resolved X-ray absorption spectroscopy in the tender X-ray range is possible.
At SwissFEL, in 2020 the Alvra endstation began with a concerted effort to develop tender X-ray spectroscopy, enabling ultrafast time-resolved measurements to complement the measurements at Phoenix: “It’s not an easy range. It’s not automatically good. We made a lot of modifications of the beamline, optimising jet systems and detectors for example,” says Bacellar. “Having Phoenix around the corner has been a great source of knowledge, and motivation for us to focus on tender X-rays.”
Forty year mystery solved
Tender X-rays were exactly what was needed to study how the C-H bond breaks in the presence of a metal catalyst, as this range includes the energy absorbed by certain electrons in the rhodium catalyst.
“The catalyst is immersed in a dense octane solution, but by taking the perspective of the metal [rhodium], we could specifically pick the one C-H bond out of hundreds of thousands which is made to break,” explains Raphael Jay, researcher at Uppsala University and first author of the paper.
With the help of theoretical interpretations of their complex experimental data, the scientists could show how electrons were exchanged between the metal catalyst and the C-H group in just the right proportion. “We can see how charge flowing from the metal onto the C-H bond glues the two chemical groups together. Charge flowing in the opposite direction instead acts as a scissor that eventually breaks the C and the H atom apart,” explains Ambar Banerjee, Postdoctoral researcher at Uppsala University and lead theoretician of the study.
The study solves a forty-year-old mystery about how an activated catalyst can break strong C-H bonds by carefully exchanging fractions of electrons without the need for huge temperatures or pressures. The next step will be to learn to direct the flow of electrons to help develop better catalysts for the chemical industry that can make efficient use of methane and other alkanes.
At Alvra, the team are pleased that their work in the tender X-ray regime is paying off. “This was one of the first experiments following our efforts to enable tender X-ray spectroscopy with good signal to noise. So, seeing the scientific output is really rewarding for us,” says Camila Bacellar.
Text: Based on a press release from Uppsala University with additions and modifications from Paul Scherrer Institute/ Miriam Arrell
© PSI provides image and/or video material free of charge for media coverage of the content of the above text. Use of this material for other purposes is not permitted. This also includes the transfer of the image and video material into databases as well as sale by third parties.
Contact
Dr. Camila Bacellar
Group Leader and Beamline Scientist at Alvra
Paul Scherrer Institute, Forschungsstrasse 111, 5232 Villigen PSI, Switzerland
Telephone: +41 56 310 42 31, e-mail: camila.bacellar@psi.ch
Dr. Thomas Huthwelker
Group Leader Phoenix Beamline
Paul Scherrer Institute, Forschungsstrasse 111, 5232 Villigen PSI, Switzerland
Telephone: +41 56 310 53 14, e-mail: thomas.huthwelker@psi.ch
Dr. Grigory Smolentsev
Beamline scientist at Operando spectroscopy
Paul Scherrer Institute, Forschungsstrasse 111, 5232 Villigen PSI, Switzerland
Telephone: +41 56 310 51 73, e-mail: grigory.smolentsev@psi.ch
Dr. Raphael Jay
Researcher at the Department of Physics and Astronomy, Uppsala University
Telephone: +46 76 022 67 04, e-mail: raphael.jay@physics.uu.se
Original Publication
Tracking C-H activation with orbital resolution
Jay et al. Science, 1. June 2023 (online)
DOI: 10.1126/science.adf8042