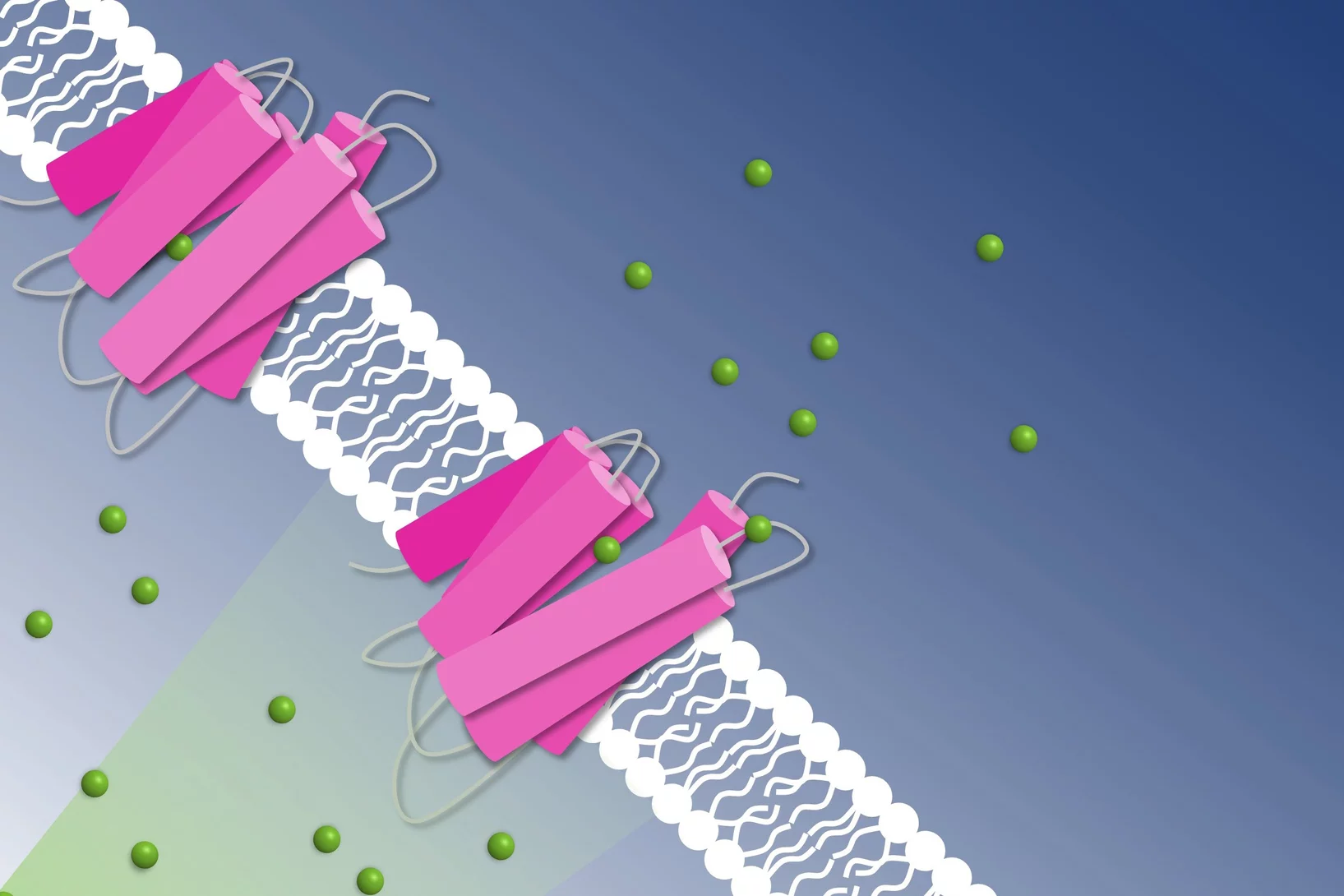
Light from the sun damages DNA. Plants, insects and many other animals have an inbuilt medic: the enzyme photolyase, which gracefully uses sunlight to repair DNA damaged by sunlight. Recently, three international user groups used SwissFEL to elucidate this mechanism, with two of the studies published in Science and a third in Nature Chemistry. This research deepens our knowledge of how life has cleverly adapted to flourish under the sun.
In the background to these discoveries are Camila Bacellar and her team at the Alvra experimental station, on the hard X-ray branch of the SwissFEL. In an interview, she tells us how Alvra has developed into a special tool for biology and chemistry research.
Camila, tell us about the recent studies with photolyase. Was it a coincidence that three separate research teams wanted to use the SwissFEL to discover how it repairs DNA?
Camila Bacellar: The SwissFEL is perfectly suited to such studies because this process of DNA repair is naturally photoactive: it begins with photolyase absorbing photons of sunlight. At the SwissFEL, we can mimic this process. We activate the photolyase with a UV laser. Then using ultrafast flashes of X-ray light from the SwissFEL, we follow how the system changes on timescales ranging from milliseconds to femtoseconds.
Two of the three groups studied photolyase with a piece of damaged DNA bound. The damaged DNA is a dimer complex, and photolyase breaks a bond to repair the DNA. The level of detail was stunning: for example, we could see subtle changes in bond angles and transient intermediate steps.
You’ve also recently had breakthroughs with other photoactive biological systems.
Indeed: for example, the protein rhodopsin. This is something that PSI itself has a lot of research experience in. Last year, in an experiment performed at Alvra, researchers from PSI revealed the first molecular events of vision, which occur within a picosecond when this protein absorbs a photon of light in the retina. We have also made breakthroughs into the mechanisms of light-driven pumps that use this protein to control ion levels in the cell.
Do you also study proteins that are not naturally photoactive?
Yes. We’re developing techniques to activate proteins without using light: in particular, with a change in temperature, or mixing.
Then, there are some proteins that aren’t naturally photoactive but have been modified to be photoactive for pharmacology. In this way, drugs are being designed that can be switched on or off by light. A research group at PSI recently enabled significant advances in this field, known as photopharmacology, using the SwissFEL.
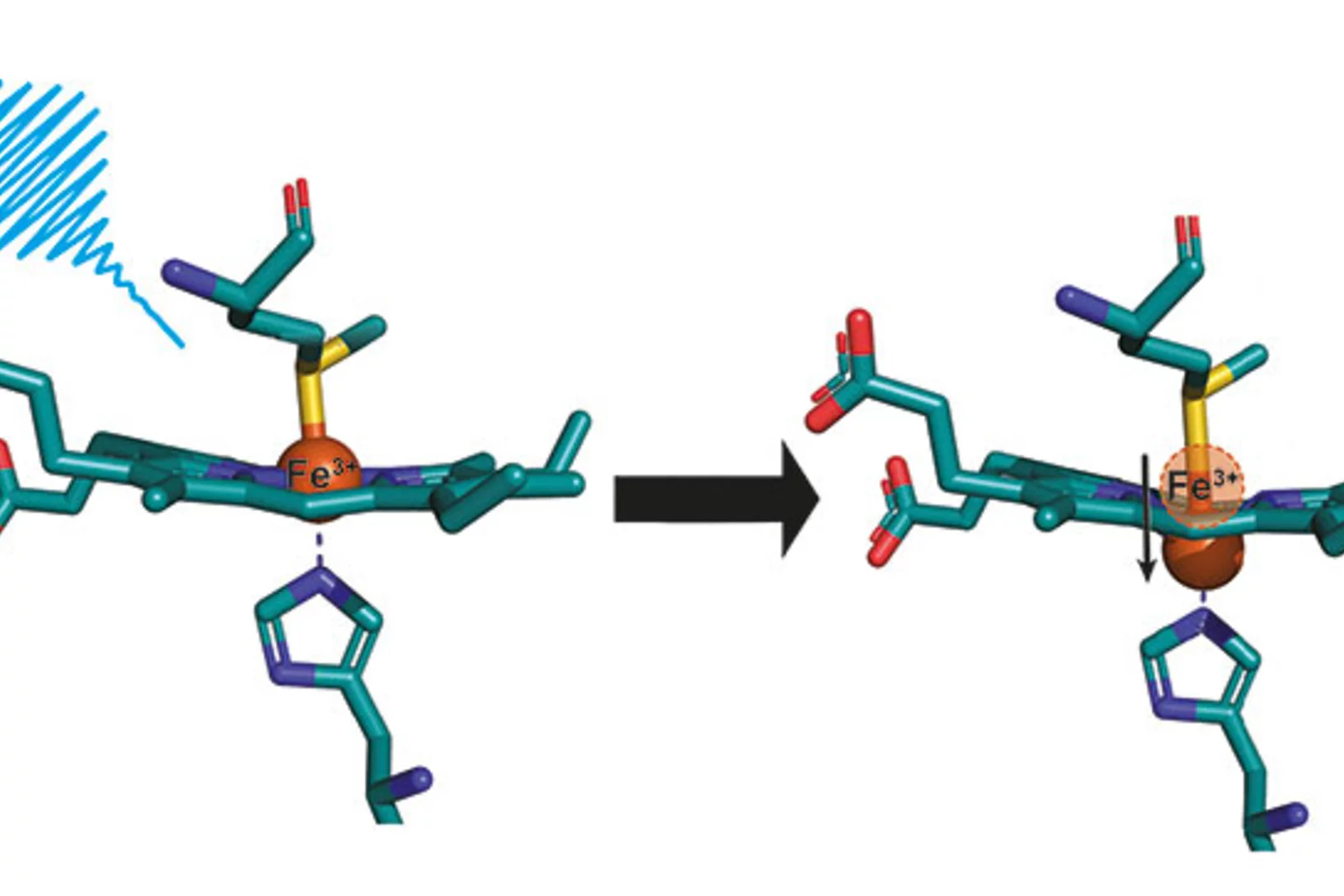
There are also some proteins that aren’t naturally photoactive, but light can be used to trigger a change mimicking the first steps in a natural process. This is the case with haem proteins, a class of proteins that perform a wide range of different biological functions, from carrying oxygen to neurotransmission. In such cases, excitation with a laser causes a ligand to detach from the haem group. It was this topic that first brought me to the SwissFEL as a user, before I joined PSI as an instrument scientist in 2020.
You’re the lead author of a publication from the very first user experiments at Alvra, concerning the haem protein, cytochrome C. Tell us about your path to these first experiments at SwissFEL.
I did my PhD in the US at Berkeley and worked at the LCLS – one of the world’s first X-ray free electron lasers (FELs). Here, I fell in love with the FEL world: particularly the beamtimes. In the lab, you are often alone with your problems, with seemingly infinite time to solve them. During beamtime, everyone comes together to get something to work under a tight time constraint.
After I finished my PhD, I wanted to continue to work with FEL science. I started as a postdoc at EPFL in the Laboratory for Ultrafast Spectroscopy, based with the SwissFEL team. The timing was fortuitous: I finished my PhD in May 2017, came to EPFL in September, and SwissFEL had first light within two months.
We submitted a proposal for the first call at Alvra, got beamtime in January 2019 and achieved these really cool results with Cytochrome C. The protein is involved in electron transport in the mitochondria of almost all eukaryotic species. We could observe how the iron-containing haem group at its centre arches – or ‘domes’ – to carry out its function.
Fascinating fact: The mystery of our missing photolyase and why we can’t drop the sunscreen
Photolyase is nearly ubiquitous across the tree of life: it’s found in all plants and across most of the animal kingdom. Strangely, the one place it’s missing is in humans and other placental mammals. It seems we lost this at some point in our evolutionary history. One theory why is that our evolutionary ancestors were nocturnal for millions of years, hiding from the hungry dinosaurs and large reptiles that ruled the earth. Because they didn’t need photolyase for such a long time, it was lost. It’s a shame, because we otherwise might be able to lounge on the beach without worrying about damage to our skin!
In the research performed at Alvra, two of the groups studied photolyase from plants, and the other from fruit flies.
How does it feel to see Alvra progress from these early experiments to the most recent results with photolyase?
Oh, it feels great! Actually, we repeated part of my original experiment last year. We wanted to test something, so I put some of my sample in, which I had in the freezer. The results were incredible: It’s night and day the quality of the data!
Sometimes we look back to 2018, the pilot year. It was so hard. We ran night shifts constantly; it was a challenge to get things to work.
Given the recent scientific output, it seems the hard work has paid off.
Totally. Beginning is always hard. As the dust settled, we began to systematically focus on specific improvements. We’ve devoted significant effort to automation and improving signal to noise, and especially to step up user support.
Now our bread and butter works extremely reliably: by this, I mean time-resolved X-ray crystallography - as used for the photolyase experiments - and also time-resolved X-ray spectroscopy. This allows users to perform experiments with huge scientific impact during what, for us, is a standard beamtime. It can still be challenging at times, and requires a lot of user support, but we know how to run these experiments very well.
In turn, this frees up time to focus on more experimental techniques that advance the capabilities at Alvra. In a few years’ time, these will become the next standard experiments, and we will push the machine further in other ways.
Of course, all this is only possible because of the incredible team at Alvra. It’s also testament to how beautifully the machine was conceptualised and built. We are all one hundred percent invested in the science and the user experience, and we truly enjoy pushing the limits of what we can do as a beamline.
What kind of things can you do now that you couldn’t before?
Tender X-rays! This is an unexplored area that we’ve developed quite unique proficiency in.
Tender X-rays have a slightly lower energy than hard X-rays. A lot of protein research uses hard X-rays that tune into a metal centre. Many proteins don’t have a metal centre but do have sulfur or phosphorous atoms. Here we have access with tender X-rays.
They are also important in chemistry. Earlier in the year, tender X-ray spectroscopy at the SwissFEL enabled an important discovery made by a user group from Uppsala, published in Science. We could reveal how a rhodium catalyst breaks the C-H bond in alkanes, one of the strongest linkages in nature. Understanding this process is important for transforming green-house gases such as methane into less harmful and potentially useful chemicals.
You mention that you’ve also invested a lot of effort into user support. Tell us more about this.
Our experiments are very complex. Using a FEL can be dominated by research groups that have been using FELs since they first began over a decade ago. By providing thorough user support, we can empower groups that have the science case but lack FEL experience. We can walk users through what equipment they should bring, how much sample is needed, and help with proposal writing, and our group has really invested in this approach.
In that regard, a great benefit is that we also have world leading research groups on site in the Laboratory of Biomolecular Research. They can share their expertise - and even equipment – from the perspective of users who know the SwissFEL inside-out. This was the case with some of the photolyase studies.
By fully supporting users, we can bring in the most exciting scientific ideas. Our team believes this is how we can grow the impact of FEL science in the long term.
Text: Paul Scherrer Institute PSI/Miriam Arrell
© PSI provides image and/or video material free of charge for media coverage of the content of the above text. Use of this material for other purposes is not permitted. This also includes the transfer of the image and video material into databases as well as sale by third parties.
Contact
Dr. Camila Bacellar Cases da Silveira
Group Leader and Beamline Scientist at Alvra
Paul Scherrer Institute PSI
+41 56 310 42 31
camila.bacellar@psi.ch
Original publication
Time-resolved crystallography captures light-driven DNA repair
Nina-Eleni Christou et al.
Science, 01.12.2023
DOI: 10.1126/science.adj4270
Visualizing the DNA repair process by a photolyase at atomic resolution
Manuel Maestre-Reyna et al.
Science, 01.12.2023
DOI: 10.1126/science.add7795
Directed ultrafast conformational changes accompany electron transfer in a photolyase as resolved by serial crystallography
Andrea Cellini et al.
Nature Chemistry, 15.01.2024
DOI: 10.1038/s41557-023-01413-9